Researchers used cloning-style techniques to reprogram skin cell nuclei into eggs, with early embryos formed.
In a pioneering proof-of-concept, scientists at Oregon Health & Science University successfully converted human skin cells into “functional” egg-like cells. The method involves transferring the nucleus of a skin cell into a donor egg cell stripped of its nucleus, then inducing it to shed extra chromosomes—a process the team calls “mitomeiosis.” Some of these lab-generated eggs were fertilized in vitro, producing early-stage embryos. However, many embryos showed chromosomal abnormalities and none developed past day six, signaling that this innovation remains in its experimental phase.
1. Scientists Create Human Egg Cells From Skin

In a world-first, researchers at Oregon Health & Science University have generated egg-like cells from human skin cells. They did this by transferring the nucleus of a skin cell into an egg cell that had its own nucleus removed, a technique similar to cloning.
The process, called nuclear transfer, was followed by chemical and cellular signals to encourage the new egg cell to mimic meiosis, the natural division that halves chromosomes. This experimental method produced “functional” egg-like cells, marking a milestone in reproductive biology even though the results remain far from clinical use.
2. The Challenge of Chromosome Counts
Human skin cells carry 46 chromosomes, while eggs must carry only 23. To make the process work, scientists had to coax the transferred nucleus to discard half its chromosomes. This step is critical because a mismatch in chromosome numbers makes reproduction impossible.
The team developed a new approach, called “mitomeiosis,” to achieve this. The method combined elements of ordinary cell division and reproductive cell division. While it worked in some cases, many reprogrammed cells showed abnormal chromosome counts. This revealed both the promise and the enormous complexity of engineering egg cells outside the human body.
3. Fertilization in the Lab
Once egg-like cells were created, the scientists attempted fertilization through in vitro techniques. Out of 82 modified eggs, only a small fraction developed into early-stage embryos, with roughly nine percent reaching the blastocyst stage, which contains about 100 cells.
Despite this progress, most embryos failed to develop normally. Many displayed chromosomal abnormalities, which prevented them from advancing beyond a few days of growth. This highlighted the technical hurdles that remain. While the study proves it is possible, the high error rate underscores how much refinement is needed before the technique could be applied in fertility clinics.
4. Proof of Concept, Not a Cure
Researchers emphasized that the findings represent a proof of concept rather than a practical fertility treatment. Although lab-generated embryos did form, they did not develop beyond six days, the point at which current laws and ethical guidelines require research to stop.
The study therefore shows potential but is far from producing babies from lab-created eggs. Scientists stressed that clinical applications are likely more than a decade away. For now, the focus is on improving accuracy, ensuring genetic stability, and understanding how to safely replicate natural egg development inside a laboratory setting.
5. Potential Future Benefits

If perfected, this breakthrough could help people who cannot produce viable eggs. This includes women whose eggs have been depleted by age, chemotherapy, or medical conditions. It could also open possibilities for same-sex couples to have genetically related children by using one partner’s skin cells.
Such applications remain speculative, but they highlight the transformative potential of the technology. Even partial success could expand fertility options. However, any use in humans will require years of testing, regulatory review, and public debate to address safety, fairness, and ethical concerns around creating eggs outside the human body.
6. Chromosomal Errors Are a Major Obstacle
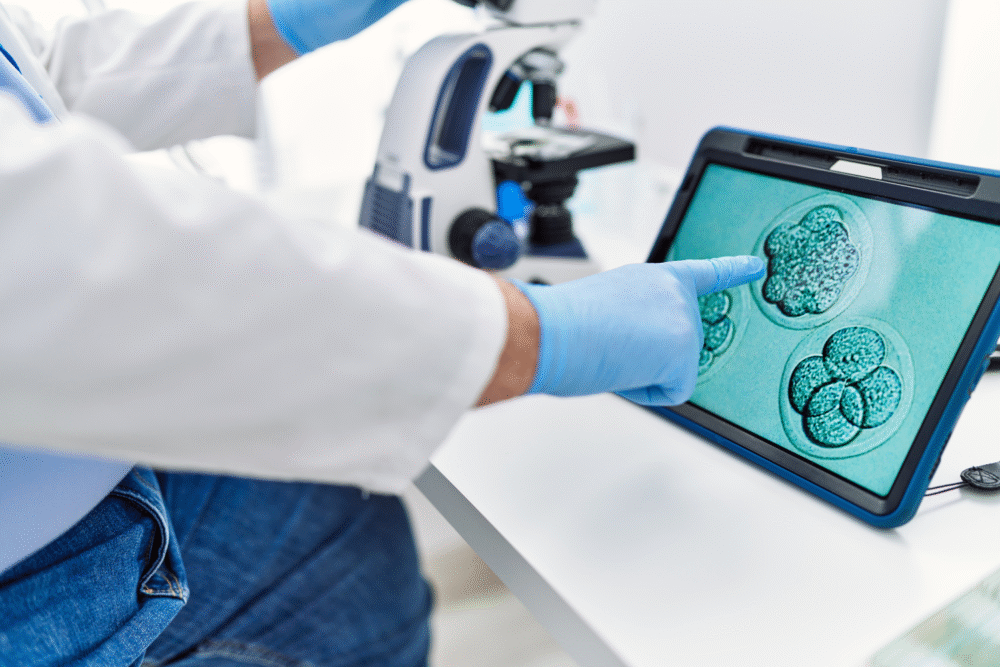
One of the biggest challenges in the study was chromosomal accuracy. Many lab-created eggs and embryos had too many or too few chromosomes, a condition known as aneuploidy. This problem prevents normal development and mirrors one of the leading causes of miscarriages in natural pregnancies.
These errors arise because the artificial process cannot yet replicate the precision of natural meiosis. Until scientists find ways to control chromosome division more reliably, the chances of producing healthy eggs remain low. This obstacle is central to moving the research from an experimental success toward any meaningful clinical application.
7. The Role of “Mitomeiosis”

The researchers coined the term “mitomeiosis” to describe their new method of inducing chromosome reduction in reprogrammed cells. It blends elements of mitosis, the everyday cell division process, with meiosis, the specialized reduction division that produces eggs and sperm.
By engineering this hybrid process, they attempted to trick the skin cell nucleus into behaving like an egg cell nucleus. While the method succeeded in some cases, it remains highly error-prone. Refining “mitomeiosis” is essential to making the process reliable. Until then, the results serve more as proof of principle than a practical tool.
8. Ethical Questions Loom Large

The creation of human egg cells from skin cells raises ethical questions as well as scientific excitement. If perfected, the technique could enable people without eggs to reproduce, but it also raises concerns about misuse, accessibility, and the limits of reproductive technology.
Regulators and ethicists are urging public discussions and oversight before clinical use moves forward. Issues such as consent, fairness, and potential “designer” applications remain unresolved. Scientists agree that while the science is groundbreaking, it must proceed with caution to ensure responsible and equitable development in line with ethical principles.
9. Echoes of Dolly the Sheep

The approach recalls cloning techniques used to create Dolly the sheep in 1996, where a nucleus from an adult cell was placed into an enucleated egg. In both cases, nuclear transfer plays a central role in reprogramming cells.
The key difference is that this research does not aim to clone entire humans. Instead, the goal is to create functional reproductive cells. By adapting methods pioneered in cloning, scientists are exploring new directions in fertility research, highlighting how advances in one field can inspire breakthroughs in another.
10. Technical Barriers Still Ahead

Beyond chromosome problems, other challenges remain. Scientists must address how gene expression resets in reprogrammed cells, how epigenetic “memories” of skin cells are erased, and whether mitochondria in donor eggs are compatible with transferred nuclei.
The complexity of the egg cell environment means that many factors must align perfectly for development to succeed. Each barrier requires new scientific solutions. Until these technical questions are answered, the process will remain limited to experimental laboratories rather than medical practice. Progress will be incremental but potentially groundbreaking over time.
11. A Scientific Milestone With Caution
Despite its limitations, the study marks a milestone in reproductive biology. For the first time, human egg-like cells were created from skin cells and fertilized in the lab. This opens new avenues for studying infertility and early human development.
But the researchers caution that clinical use is far away. High failure rates, ethical concerns, and unknown long-term effects mean the technology must be refined for years before it can move forward. Still, the study represents a dramatic step in reimagining what might be possible in human fertility science.
